Chapter 8 review chemical equations and reactions – Embarking on Chapter 8: Chemical Equations and Reactions, this discourse delves into the fundamental principles governing chemical transformations. By exploring the intricate interplay of reactants, products, and energy, we unravel the mechanisms that orchestrate the symphony of chemical reactions.
As we delve deeper into the chapter, we will dissect the art of balancing chemical equations, deciphering the language of chemical reactions. We will unravel the diverse types of reactions, each with its unique characteristics and patterns. Stoichiometry and mole calculations will empower us to predict the quantities of reactants and products involved in these reactions.
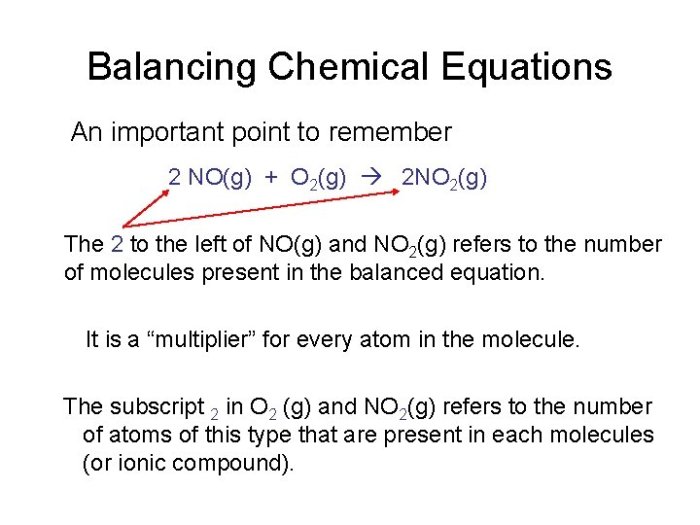
Balancing Chemical Equations: Chapter 8 Review Chemical Equations And Reactions

Balancing chemical equations involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation. This ensures that the law of conservation of mass is upheld, as atoms cannot be created or destroyed during a chemical reaction.
To balance an equation, follow these steps:
- Start by identifying the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the coefficients in front of each chemical formula until the number of atoms of each element is the same on both sides.
- Check your work by making sure that the coefficients are in the lowest whole-number ratio.
For example, consider the unbalanced equation:
Fe + HCl → FeCl 2+ H 2
To balance this equation, we can start by counting the number of atoms of each element on both sides:
Reactants: 1 Fe, 2 H, 1 Cl
Products: 1 Fe, 2 Cl, 2 H
We can see that the number of atoms of Fe and Cl is the same on both sides, but the number of atoms of H is not. To balance the equation, we can adjust the coefficient in front of H 2to 2:
Fe + 2HCl → FeCl 2+ 2H 2
Now, we can count the number of atoms of each element again:
Reactants: 1 Fe, 4 H, 2 Cl
Products: 1 Fe, 4 H, 2 Cl
The equation is now balanced.
FAQ Guide
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element is the same on both sides of the equation, reflecting the law of conservation of mass.
How do I determine the limiting reactant in a reaction?
The limiting reactant is the reactant that is completely consumed in a reaction, limiting the amount of product that can be formed. To identify the limiting reactant, compare the mole ratios of the reactants to their stoichiometric coefficients.
What factors influence the rate of a chemical reaction?
The rate of a chemical reaction is affected by factors such as concentration, temperature, surface area, and the presence of a catalyst.