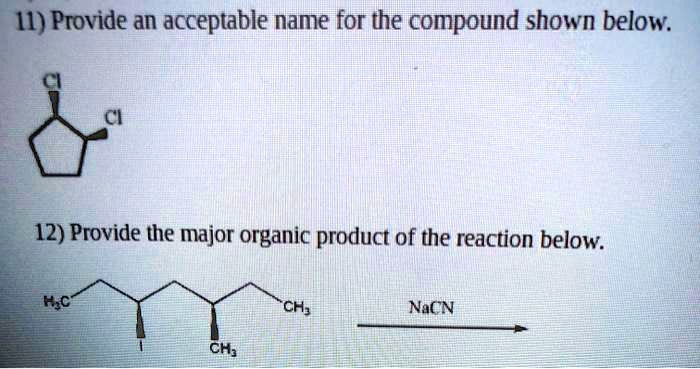
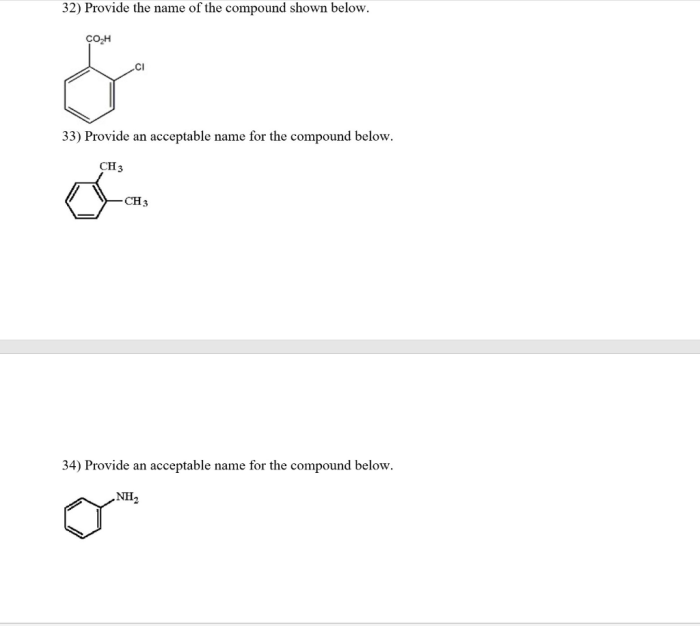
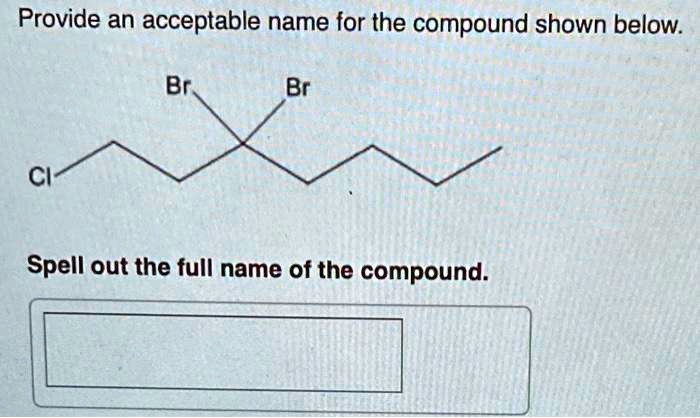
Provide an acceptable name for the compound below. – In the realm of chemistry, the ability to accurately name compounds is essential for effective communication and understanding. This guide provides a comprehensive overview of the International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules, empowering you to confidently assign acceptable names to organic compounds.
Understanding IUPAC nomenclature involves grasping the significance of prefixes, suffixes, and root words, as well as recognizing exceptions and special cases. By identifying functional groups, determining the parent chain, and naming substituents, you can systematically construct IUPAC-compliant names.
Nomenclature of Organic Compounds
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of rules for naming organic compounds. These rules provide a systematic way to assign unique and unambiguous names to organic molecules.
Understanding the Nomenclature
IUPAC nomenclature is based on three main principles:
- The parent chain is the longest continuous chain of carbon atoms in the molecule.
- The functional group is the group of atoms that gives the molecule its characteristic chemical properties.
- The substituents are the atoms or groups of atoms that are attached to the parent chain.
Identifying Functional Groups
Functional groups are specific arrangements of atoms that impart characteristic chemical properties to organic molecules. Some common functional groups include:
- Alkanes: Contain only carbon and hydrogen atoms, with all carbon atoms bonded to each other by single bonds.
- Alkenes: Contain at least one carbon-carbon double bond.
- Alkynes: Contain at least one carbon-carbon triple bond.
- Alcohols: Contain a hydroxyl group (-OH) bonded to a carbon atom.
- Carboxylic acids: Contain a carboxyl group (-COOH) bonded to a carbon atom.
Determining the Parent Chain
The parent chain is the longest continuous chain of carbon atoms in the molecule. To determine the parent chain, follow these steps:
- Identify the functional group.
- Find the longest carbon chain that contains the functional group.
- Number the carbon atoms in the parent chain, starting from the carbon atom that is attached to the functional group.
Naming the Substituents
Substituents are the atoms or groups of atoms that are attached to the parent chain. To name the substituents, use the following prefixes:
- Methyl (Me): -CH 3
- Ethyl (Et): -CH 2CH 3
- Propyl (Pr): -CH 2CH 2CH 3
- Butyl (Bu): -CH 2CH 2CH 2CH 3
Assembling the Name
To assemble the name of an organic compound, combine the name of the parent chain, the names of the substituents, and the name of the functional group. Use hyphens and commas to separate different parts of the name.
Special Cases
There are some special cases where IUPAC rules may not apply. These cases include:
- Compounds with multiple functional groups
- Compounds with complex structures
Examples and Practice, Provide an acceptable name for the compound below.
Here are some examples of organic compounds and their IUPAC names:
| Compound | IUPAC Name |
|---|---|
| CH3CH2CH2CH3 | Butane |
| CH3CH2CH=CH2 | 1-Butene |
| CH3CH2CH2OH | 1-Propanol |
| CH3COOH | Acetic acid |
Practice naming organic compounds using the IUPAC rules. The more you practice, the easier it will become.
FAQ Guide: Provide An Acceptable Name For The Compound Below.
What are the key principles of IUPAC nomenclature?
IUPAC nomenclature follows specific rules to name compounds based on their structure, including identifying the parent chain, prefixes for substituents, and suffixes for functional groups.
How do I determine the parent chain in a compound?
The parent chain is the longest continuous chain of carbon atoms in the molecule, which may contain functional groups or other substituents.
What is the significance of functional groups in naming compounds?
Functional groups are specific arrangements of atoms within a molecule that impart characteristic chemical properties and determine the suffix used in the IUPAC name.